Author Archives: admin
Strongest company in Estonia 2024
- 25 May, 2024
- Posted by admin
- 0 Comment(s)
MasterChem OÜ Demonstrates Strong Financial Health and Strategic Expansion in the Chemical Industry
Maardu, Harjumaa – MasterChem OÜ, a leading wholesaler of chemical products, has consistently demonstrated strong financial stability and strategic growth, as evidenced in the latest comprehensive report by Creditinfo Eesti AS. The company, which is headquartered in Maardu, boasts a robust financial position with a notable credit rating of ‘A’ and a very low insolvency probability of 1.4%.
Established in 2001, MasterChem OÜ has grown into a key player in the chemical products sector. The company specializes in the wholesale, and manufacture of various chemical products, including detergents and other cleaning agents. Over the years, it has expanded its operations to include non-specialised wholesale trade and other technical activities.
In 2023, MasterChem OÜ reported a net sales of €1,557,892, maintaining a significant portion of its revenue from exports, which amounted to €106,212. Despite a slight decline in net sales by 2.4% compared to the previous year, the company achieved a remarkable increase in annual profit, reaching €106,212, up 61.5% from the previous year.
The company’s commitment to maintaining a strong balance sheet is evident in its total assets, which increased by 39.9% from the previous year to €1,525,631. This growth is supported by significant investments in tangible assets and careful management of inventory and receivables.
MasterChem OÜ has maintained a consistent policy regarding its capital structure and credit management, which has allowed it to operate with a high degree of financial independence from debt capital. Its equity, amounting to €883,033, represents a significant 57.9% of its total liabilities and equity, underscoring the company’s solid financial foundation.
Further emphasizing its financial prudence, MasterChem OÜ has no outstanding claims or payment defaults as of May 2024, maintaining a clean slate with the Estonian Tax and Customs Board.
As MasterChem OÜ continues to navigate the complexities of the global market, its strategic focus on sustainable growth, financial health, and robust operational strategies positions it as a resilient and forward-looking leader in the industry.
Study of the thermophysical dynamics of aqueous solutions of glycerol in heating systems development of commercial products Master BIO-10;-20;-30;-40PRO™
- 19 Mar, 2024
- Posted by admin
- 0 Comment(s)
Abstract:
This study examines the unique thermophysical properties of aqueous glycerol solutions with a focus on their application in heating systems. By examining variables such as density, viscosity, thermal conductivity, and specific heat capacity at various glycerol concentrations and temperatures, we offer insight into the potential applications and limitations of Master BIO products in thermal engineering.
Introduction:
Aqueous glycerin solutions are known for their diverse applications, owed largely to their distinct physical properties. This research provides a comprehensive analysis of these properties, particularly in the context of heating systems.
Methodology:
We analyzed glycerin-water mixtures with glycerin concentrations ranging from 10% to 70% by mass, at temperatures between 0°C and 100°C. Data was collected on density, dynamic viscosity, thermal conductivity, and specific heat capacity.
Results:
Freezing and Boiling Points: The freezing point shows a sharp decrease at higher glycerin concentrations, beneficial for low-temperature applications.
Glycerine and water solution – Freezing and Boiling Points
| Glycerine to Water Concentration (% by mass, weight) | Freezing Point | Boiling Point (at normal pressure) |
| t°C | t°C | |
| 98.2 | 13.3 | 290 |
| 95 | 7.8 | 167 |
| 90 | -1.7 | 138 |
| 80 | -20.6 | 121 |
| 70 | -38.9 | 114 |
| 66.7 | -46.1 | 112 |
| Master BIO-40PRO™ 70 | -40.2* | 110 |
| 60 | -34.4 | 109 |
| Master BIO-30PRO™ 57 | -30.6* | 108 |
| 50 | -22.8 | 106 |
| Master BIO-20PRO™ 47 | -20.0* | 105 |
| 40 | -15.6 | 104 |
| Master BIO-10PRO™ 31 | -10.5* | 102 |
| 30 | -9.4 | 103 |
| 20 | -5.0 | 101 |
| 10 | -1.7 | 101 |
| *+_0.5 depends on the inhibitor additive |
Density and Viscosity: Increasing glycerin concentration leads to higher density and viscosity, impacting flow and heat transfer.
Density of aqueous glycerol solution
The density of a mixture of glycerin and water is given in the table for glycerin concentrations from 10 percent to 70 percent by weight in the temperature range from zero to one hundred degrees Celsius.
| Temperature | 10% | 20% | 30% | 31% Master BIO-10PRO™ 31 | 40% | 47% Master BIO-20PRO™ | 50% | 57,% Master BIO-30PRO™ | 60% | 70% Master BIO-40PRO™ |
| оС | ρ, g/cm3 | ρ, g/cm3 | ρ, g/cm3 | ρ, g/cm3 | ρ, g/cm3 | ρ, g/cm3 | ρ, g/cm3 | ρ, g/cm3 | ρ, g/cm3 | ρ, г/см3 |
| 0 | 1,025 | 1,052 | 1,079 | 1,072 | 1,107 | 1,118 | 1,135 | 1,154 | 1,163 | 1,192 |
| 20 | 1,022 | 1,047 | 1,073 | 1.075 | 1,099 | 1,113 | 1,126 | 1,145 | 1,154 | 1,181 |
| 40 | 1,016 | 1,039 | 1,064 | 1.066 | 1,089 | 1,107 | 1,115 | 1,134 | 1,142 | 1,169 |
| 60 | 1,006 | 1,03 | 1,053 | 1,056 | 1,078 | 1,095 | 1,103 | 1,122 | 1,13 | 1,156 |
| 80 | 0,994 | 1,017 | 1,041 | 1,042 | 1,066 | 1,083 | 1.091 | 1,110 | 1,117 | 1.144 |
| 100 | 0,982 | 1,004 | 1,027 | 1.029 | 1,052 | 1,069 | 1,077 | 1,095 | 1,104 | 1,302 |
Dynamic viscosity of an aqueous glycerol solution
The viscosity of an aqueous solution of glycerin is given in the table in the range of mixture temperatures from zero to one hundred degrees Celsius and glycerin concentration from 10% to 70%. It is noteworthy that adding only 10% (by weight) glycerol to water can increase the dynamic viscosity of the solution by ~30%.
Viscosity of aqueous glycerol solution (content in percent by weight)
| Temperature | 10% | 20% | 30% | 31% Master BIO-10PRO™ | 40% | 47% Master BIO-20PRO™ | 50% | 57,% Master BIO-30PRO™ | 60% | 70% Master BIO-40PRO™ |
| оС | μ Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 | μ,Pa*10-3 |
| 0 | 2,44 | 3,44 | 5,14 | 5,53 | 8,25 | 12,69 | 14,60 | 25,37 | 29,90 | 76,00 |
| 20 | 1,31 | 1,76 | 2,50 | 2,62 | 3,72 | 5,32 | 6,00 | 9,37 | 10,80 | 22,50 |
| 40 | 0,83 | 1,07 | 1,46 | 1,52 | 2,07 | 2,79 | 3,10 | 4,48 | 5,08 | 9,40 |
| 60 | 0,58 | 0,73 | 0,96 | 1,09 | 1,30 | 1,69 | 1,86 | 2,55 | 2,85 | 4,86 |
| 80 | – | – | 0,69 | 0,71 | 0,92 | 1,15 | 1,25 | 1,66 | 1,84 | 2,90 |
| 100 | – | – | – | – | 0,67 | 0,84 | 0,91 | 1,17 | 1,28 | 1,93 |
Thermal Conductivity: Higher glycerin concentrations result in lower thermal conductivity, suggesting limitations in heat transfer applications.
The thermal conductivity values of an aqueous glycerol solution are shown in the table for a temperature range from 20 to 80 degrees Celsius and glycerol concentration from 10% to 70%. With increasing glycerol concentration, the thermal conductivity of the aqueous solution decreases. With a glycerol content of 50%, the thermal conductivity of the mixture is ~30% less than that of pure water.
| Temperature | 10% | 20% | 30% | 31% Master BIO-10PRO™ 31 | 40% | 47% Master BIO-20PRO™ | 50% | 57,% Master BIO-30PRO™ | 60% | 70% Master BIO-40PRO™ |
| оС | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) | W/(cm•oC) |
| 20 | 0,56 | 0,52 | 0,48 | 0,48 | 0,45 | 0.42 | 0,41 | 0,39 | 0,38 | 0,35 |
| 40 | 0,59 | 0,54 | 0,50 | 0,5 | 0,46 | 0,43 | 0,42 | 0,40 | 0,39 | 0,36 |
| 60 | 0,61 | 0,57 | 0,52 | 0,52 | 0,48 | 0,45 | 0,44 | 0,41 | 0,39 | 0,36 |
| 80 | 0,64 | 0,59 | 0,54 | 0,54 | 0,49 | 0,46 | 0,45 | 0,42 | 0,40 | 0,36 |
Specific Heat Capacity: The capacity decreases with increased glycerin concentration, affecting the energy efficiency of heating systems.
Estimates of the heat capacity of an aqueous glycerol solution are given in the table for temperatures from 20 to 80 degrees Celsius and glycerol concentrations from 10 to 70 percent. With increasing glycerol concentration, the thermal conductivity of the solution decreases. Under normal conditions and a content of 10% glycerol, the heat capacity of the mixture is ~2 times less than the heat capacity of pure water.
Heat capacity of a mixture of glycerin with water (content in percent by weight)
| Temperature | 10% | 20% | 30% | 31% Master BIO-10PRO™ 31 | 40% | 47% Master BIO-20PRO™ | 50% | 57,% Master BIO-30PRO™ | 60% | 70% Master BIO-40PRO™ |
| оС | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) | kJ/(kg•oC) |
| 20 | 1,998 | 1,907 | 1,816 | 1,807 | 1,725 | 1,716 | 1,634 | 1,625 | 1,542 | 1,452 |
| 40 | 2,002 | 1,916 | 1,830 | 1,210 | 1,744 | 1,735 | 1,659 | 1,650 | 1,573 | 1,487 |
| 60 | 2,010 | 1,929 | 1,848 | 1,840 | 1,767 | 1,758 | 1,687 | 1,679 | 1,606 | 1,525 |
| 80 | 2,024 | 1,948 | 1,871 | 1,864 | 1,795 | 1,786 | 1,718 | 1,710 | 1,642 | 1,608 |
Discussion:
The study highlights the trade-offs between glycerin concentration and thermophysical properties. While higher concentrations offer lower freezing points, they also reduce thermal conductivity and specific heat capacity, potentially limiting efficiency in certain heating applications.
Conclusion:
Aqueous glycerin solutions present a viable option for specialized heating systems, particularly in environments with varying temperatures. However, careful consideration of glycerin concentration is crucial to balance between antifreeze properties and heat transfer efficiency.
Procedure in case of emergency shutdown of the heating system.
Solutions of glycerin and water at zero temperatures and below have a high level of viscosity. Therefore, if the system has cooled down, it is advisable to first turn on the heating elements themselves, and then start circulation. This will reduce the load on the pumps.
© 2024 Sergei Vesselkov. All rights reserved.
All rights are reserved and no part of the publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without your prior written permission.
The concentration of Master ECO-10;-20;-30;-40;-50PRO™ solutions and their effect on the thermophysical properties of aqueous solutions of propylene glycol for heating systems and fire protection systems.
- 27 Feb, 2024
- Posted by admin
- 0 Comment(s)
Water, a fundamental element in various industrial processes, serves as the primary means for heat transfer due to its unique thermophysical properties. However, the limitation associated with the freezing point of water requires the use of alternative fluids, such as antifreeze solutions, in specific scenarios.
The superiority of water as a means of heat transfer:
Water is an outstanding heat transfer fluid in industrial systems due to its high thermal conductivity and heat capacity. These properties allow for efficient heat absorption and transfer, making water an ideal medium in systems that require rapid temperature control. In addition, the low viscosity of water ensures smooth flow through pipes and channels, increasing system efficiency. Low thermal expansion reduces the risk of damage due to temperature changes. Moreover, water is readily available, inexpensive, and environmentally friendly, making it the preferred choice in many industrial applications.
Water limitation – freezing point:
Despite these benefits, water has a critical disadvantage – its freezing point at 0°C. This limitation poses a significant problem in environments exposed to sub-zero temperatures, as frozen water can cause system failure or damage.
The need for antifreeze solutions in industrial processes:
To overcome the limitation of water’s freezing point, the industry is turning to antifreeze solutions. They are vital in the food processing sector, where processes often require refrigeration. Likewise, in pharmaceutical manufacturing, maintaining specific temperatures is critical to product stability and effectiveness.
In the food and pharmaceutical industries, as well as in fire extinguishing systems, harmless aqueous solutions based on propylene glycol or glycerin are used. Solar collectors use propylene glycol-based fluids.
Natural solutions are also used in heating systems of private homes, children’s institutions, and industrial enterprises, where it is necessary to minimize the risk of harm if solutions enter the drinking system. Solutions based on propylene glycols and glycerin are safe compared to ethylene glycol solutions.
Aqueous solutions of propylene glycol developed by the MasterChem laboratory are divided into groups according to freezing point and rust protection.
Master ECO-20PRO™
Master is a patented product of MasterChem
ECO – means an aqueous solution based on propylene glycol. (Other MasterChem coolant ranges have meanings
BIO – solution of glycerin and water
EWS – ethylene glycol and water solution
-10; -20; -30; -40; -50 freezing point of aqueous solutions Celsius
NOR – aqueous solution without rust additives
PRO – aqueous solution with anti-rust additive – contains an inhibitor
for example, Master ECO-20PRO™ – an aqueous solution based on propylene glycol, does not freeze at temperatures down to -20C and contains inhibitor additives
The properties of aqueous propylene glycol solutions are indeed critical to their use as a heat transfer fluid in various applications. Let’s discuss these properties in more detail:
– Density: Aqueous solutions of propylene glycol have a higher density than water, usually 6-8% higher. This density increases with increasing propylene glycol concentration. The higher density of these solutions can affect the flow and heat transfer characteristics of systems where they are used.
– Specific Heat and Thermal Conductivity: Both the specific heat and thermal conductivity of propylene glycol solutions are lower than that of water and decrease by up to 20% with increasing propylene glycol concentration. This reduction becomes more significant at lower temperatures, especially in sub-zero conditions. This means that the solution’s ability to retain and transfer heat is reduced compared to water.
– Viscosity: Both kinematic and dynamic viscosity of aqueous solutions of propylene glycol are higher than that of water, approximately 4-5 times higher at positive temperatures. When the propylene glycol concentration is increased to about 55% (which is usually the practical limit), the viscosity can increase by a factor of 10-15, especially when the crystallization temperature drops to about -40 °C. High viscosity at lower temperatures can affect the pumpability and fluidity of the solution, which is a critical factor in system design and operation.
– These properties make aqueous solutions of propylene glycol especially useful in applications where freezing temperatures may occur, such as in HVAC systems, refrigeration, and various industrial processes. The formulation of these solutions is a balance between the required antifreeze properties and the desired heat transfer characteristics.
– It is important for engineers and technicians to consider these properties when designing and operating systems that use aqueous propylene glycol solutions to ensure optimal performance and efficiency.
The increased viscosity of aqueous solutions of propylene glycol at negative operating temperatures actually leads to significant hydraulic losses due to friction in pipelines and overcoming hydraulic resistance in all components of cooling and industrial air conditioning systems. This factor is critical in system design and operation because higher-viscosity fluids require more energy to pump, resulting in increased operating costs and potential system stress.
In addition, a decrease in the specific heat capacity and thermal conductivity of propylene glycol solutions by up to 20% compared to water requires adjustments to the operation of the system. To ensure the transfer of the required thermal power (energy), it may be necessary to increase the coolant circulation rate or other technical solutions. This can affect the efficiency of heat transfer processes and the overall performance of heating and cooling systems.
These factors become especially important in various climates where extreme temperatures can significantly affect fluid properties. When designing and operating heating and industrial air conditioning systems, engineers must consider these characteristics of propylene glycol solutions. This includes consideration of alternative system designs, such as larger pumps or pipes, to account for increased fluid viscosity and reduced heat transfer efficiency.
Thus, the use of aqueous propylene glycol solutions at extreme temperatures requires careful consideration of their thermophysical properties to ensure efficient and reliable operation of the system. This may involve a detailed analysis of system parameters and potentially more complex or expensive system designs to effectively address these issues.
Master ECO-10PRO™ Thermophysical properties of a 25% aqueous solution of propylene glycol, freezing point minus – 10°C
| Solution temperature, t°C | Density, kg/m**3 | Heat capacity, Average, kJ/(kg*K) | Thermal conductivity, W/(m*K) | Dynamic viscosity, *10-3[N*s/m**2] | Kinematic viscosity, *10-6[(m**2/s] |
| -10°C | 1028 | 3.92 | 0.465 | 10,231 | 9.81 |
| 0°C | 1025 | 3.95 | 0.470 | 6,180 | 6.02 |
| 20°C | 1019 | 3.98 | 0.478 | 2,860 | 2.81 |
| 40°C | 1011 | 4.00 | 0.491 | 1.421 | 1.40 |
| 60°C | 998 | 4.03 | 0.505 | 0.903 | 0.90 |
| 80°C | 981 | 4.05 | 0.519 | 0.671 | 0.68 |
| 100°C | 973 | 4.08 | 0.533 | 0.509 | 0.52 |
Master ECO-20PRO™ Thermophysical properties of 37% aqueous solution of propylene glycol, crystallization temperature minus – 20°C
| Solution temperature, t°C | Density, kg/m**3 | Heat capacity, Average, kJ/(kg*K) | Thermal conductivity, W/(m*K) | Dynamic viscosity, *10-3[N*s/m**2] | Kinematic viscosity, *10-6[(m**2/s] |
| -20°C | 1051 | 3.68 | 0.420 | 47.25 | 45.10 |
| 0°C | 1045 | 3.72 | 0.425 | 12.54 | 12.12 |
| 20°C | 1036 | 3.77 | 0.429 | 4,562 | 4.41 |
| 40°C | 1025 | 3.82 | 0.433 | 2,261 | 2.23 |
| 60°C | 1012 | 3.88 | 0.437 | 1,320 | 1.30 |
| 80°C | 997 | 3.93 | 0.441 | 0.897 | 0.91 |
| 100°C | 982 | 4.00 | 0.445 | 0.687 | 0.71 |
Master ECO-30PRO™ Thermophysical properties of 45% aqueous solution of propylene glycol, crystallization temperature minus – 30°C
| Solution temperature, t°C | Density, kg/m**3 | Heat capacity, Average, kJ/(kg*K) | Thermal conductivity, W/(m*K) | Dynamic viscosity, *10-3[N*s/m**2] | Kinematic viscosity, *10-6[(m**2/s] |
| -30°C | 1066 | 3.45 | 0.397 | 160.2 | 150 |
| -20°C | 1062 | 3.49 | 0.396 | 74.3 | 70 |
| -10°C | 1058 | 3.52 | 0.395 | 31.74 | 30 |
| 0°C | 1054 | 3.56 | 0.395 | 18.97 | 18 |
| 20°C | 1044 | 3.62 | 0.394 | 6,264 | 6 |
| 40°C | 1033 | 3.69 | 0.393 | 2,978 | 2.9 |
| 60°C | 1015 | 3.76 | 0.391 | 1.624 | 1.6 |
| 80°C | 999 | 3.82 | 0.391 | 1.10 | 1.1 |
| 100°C | 984 | 3.89 | 0.390 | 0.807 | 0.82 |
Master ECO-40PRO™ Thermophysical properties of 53% aqueous solution of propylene glycol, crystallization temperature minus – 40°C
| Solution temperature, t°C | Density, kg/m**3 | Heat capacity, Average, kJ/(kg*K) | Thermal conductivity, W/(m*K) | Dynamic viscosity, *10-3[N*s/m**2] | Kinematic viscosity, *10-6[(m**2/s] |
| -40°C | 1069 | 3.15 | 0.373 | 212.0 | 215 |
| -30°C | 1068 | 3.22 | 0.374 | 180.5 | 180 |
| -20°C | 1066 | 3.26 | 0.376 | 83.3 | 80 |
| -10°C | 1052 | 3.29 | 0.375 | 39.74 | 40 |
| 0°C | 1058 | 3.33 | 0.375 | 22.67 | 23 |
| 20°C | 1046 | 3.39 | 0.374 | 7,534 | 8 |
| 40°C | 1038 | 3.46 | 0.378 | 3.025 | 3 |
| 60°C | 1021 | 3.53 | 0.384 | 1.814 | 1.9 |
| 80°C | 1014 | 3.59 | 0.385 | 1.21 | 1.3 |
| 100°C | 992 | 3.66 | 0.390 | 0.843 | 0.85 |
Master ECO-50PRO™ Thermophysical properties of 60% aqueous solution of propylene glycol, crystallization temperature minus – 50°C
| Solution temperature, t°C | Density, kg/m**3 | Heat capacity, Average, kJ/(kg*K) | Thermal conductivity, W/(m*K) | Dynamic viscosity, *10-3[N*s/m**2] | Kinematic viscosity, *10-6[(m**2/s] |
| -50°C | 1071 | Not Tested | Not Tested | Not Tested | Not Tested |
| -40°C | 1070 | 3.12 | 0.353 | 243.0 | 245 |
| -30°C | 1069 | 3.20 | 0.354 | 195.5 | 196 |
| -20°C | 1067 | 3.24 | 0.355 | 92.3 | 93 |
| -10°C | 1054 | 3.27 | 0.324 | 41,2 | 42 |
| 0°C | 1059 | 3.30 | 0.355 | 24.3 | 25 |
| 20°C | 1047 | 3.36 | 0.353 | 8.2 | 9 |
| 40°C | 1039 | 3.44 | 0.357 | 3.87 | 4 |
| 60°C | 1023 | 3.51 | 0.363 | 1.886 | 1.9 |
| 80°C | 1017 | 3.57 | 0.364 | 1.38 | 1.4 |
| 100°C | 998 | 3.64 | 0.370 | 0.874 | 0.88 |
The freezing point of aqueous solutions of propylene glycol is indeed an important thermophysical parameter, especially in cases where low temperatures are encountered. The relationship between propylene glycol concentration and freezing point is nonlinear.
Dependence of freezing temperature on concentration. As the concentration of propylene glycol in water increases, the freezing point of the solution decreases. This decrease in freezing point is nonlinear, meaning it does not decrease at a constant rate as concentration increases.
Practical minimum freezing point: At a propylene glycol concentration of about 70%, the solution reaches a practical minimum freezing point of about -58°C. A further increase in concentration, up to 98%, only slightly reduces the freezing point to approximately -60°C.
Standard Concentrations: Typically, propylene glycol solutions are used in standard concentrations of 30–40%. This range is considered optimal for balancing the properties of antifreeze and other physical characteristics of the solution.
Cost Considerations: The cost of the coolant largely depends on the concentration of propylene glycol. The use of concentrations above 70% is generally not considered economical due to the minimal reduction in freezing point beyond this concentration.
Density at 20°C: The density of propylene glycol solutions also varies depending on the concentration and is usually calculated at a standard temperature of 20°C. Higher concentrations of propylene glycol result in higher densities.
These properties are critical to the design and operation of systems that use propylene glycol as a heat transfer fluid, especially in variable or low-temperature environments. Engineers need to understand these relationships to select the appropriate concentration for their specific application and ensure system efficiency and cost-effectiveness.
Effect of clear propylene glycol concentration on the freezing point of an aqueous solution. Density of the solution at a temperature of 20°C.
| Propylene glycol concentration, % | Freezing point (beginning of crystallization), t°C | Density at 20°C |
| 31% | -15 °C | 1.022 |
| 36% | -20 °C | 1.028 |
| 42% | -25 °C | 1.031 |
| 45% | -30 °C | 1.035 |
| 50% | -35 °C | 1.038 |
| 55% | -45 °C | 1,040 |
| 60% | -55 °C | 1,042 |
| 65% | -57 °C | 1,043 |
| 70% | -58 °C | 1,044 |
The service life of Master ECO coolants is at least 7 years. The price of the coolant depends on the concentration of the main base component. When ordering and purchasing it, it is necessary to take into account the percentage of propylene glycol in the coolant brand. The temperature range in which the all-season low-freezing coolant will be in a working condition depends on this indicator.
Procedure in case of emergency shutdown of the heating system.
Solutions of propylene glycol and water at zero temperatures and below have a high level of viscosity. Therefore, if the system has cooled down, it is recommended to first turn on the heating elements themselves, and then start circulation. This will reduce the load on the pumps.
© 2024 Sergei Vesselkov. All rights reserved.
All rights are reserved and no part of the publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without your prior written permission.
How to calculate the volume of the heating system
- 27 Feb, 2024
- Posted by admin
- 0 Comment(s)
| kW | |
| liters | |
| liters (calc. filling 25%) |
| Pipe diameter, mm | Pipe length, m |
|---|---|
| 16х2.0 | |
| 20х2.0 | |
| 26х3.0 | |
| 32х3.0 | |
| 20х3.4 | |
| 25х4.2 | |
| 32х5.4 | |
| 40х6.7 |
Radiators: liters
Boiler, collectors, fittings: liters
Expansion tank: liters
Pipes: liters
Total: liters
Aqueous solution of coolant and coolant for heating and cooling systems based on propylene glycol
- 27 Feb, 2024
- Posted by admin
- 0 Comment(s)
Aqueous solution of propylene glycol – aqueous solution of coolant and antifreeze for heating and cooling systems
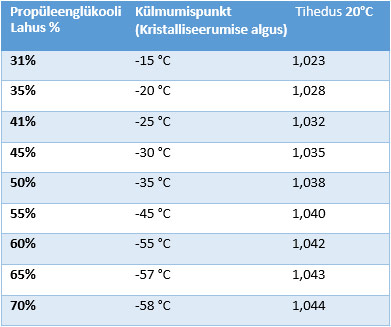
Dependence of propylene glycol concentration on the freezing point of aqueous solution (onset of crystallization). Solution density values at 20 ° C.
| Propylene Glycol Solution % | Freezing point (onset of crystallization), t°C | Density 20°C |
|---|---|---|
| 31% | -15°C | 1,023 |
| 35% | -20°C | 1,028 |
| 41% | -25°C | 1,032 |
| 45% | -30°C | 1,035 |
| 50% | -35°C | 1,038 |
| 55% | -45°C | 1,040 |
| 60% | -55°C | 1,042 |
| 65% | -57°C | 1,043 |
| 70% | -58°C | 1,044 |
Answers to frequently asked questions about biofireplaces and biofuels
- 27 Feb, 2024
- Posted by admin
- 0 Comment(s)
Answers to frequently asked questions about biofireplaces and biofuels
Do bio fireplaces heat a room?
– Yes
Can be used as the main heat source
– no, it is more expensive than traditional heat sources and requires frequent maintenance (bioethanol replenishment)
Can be used as a temporary/emergency heat source
– yes
The news often talks about a possible power outage – many people ask themselves – what to do, how to heat the room?
If it is not possible to use wood heating or you live in an apartment – then this is the solution for you.
You can install a bio fireplace on the street, in a house, in an apartment, in an office, in a workshop, in a cafe and in other public places.
Biofireplace as a heat source:
To answer the question of how well bio fireplaces heat a room, you need to carefully study the characteristics of a particular model and take into account the area of the room.
Standard small models, when consuming one liter of biofuel, provide power up to 5-6 kW. For every 10 square meters, the power requirement is about 1 kW per hour. It follows that in this case it is enough to burn half a liter of bioethanol in 60 minutes.
Now let’s examine it from an economic point of view. One liter of bioethanol XXX costs 2.20 liters when bought in a large package. Accordingly, the cost of one kilowatt of heat costs 0.44 euros. The cost is 2 times more expensive than the current electricity general service package, but it is an independent heating source.
How long does the biofireplace burn?
Depending on the size of the tank, 1 liter of fuel (on small models) burns out in 3-4 hours.
What is the efficiency of bio fireplaces – 95%
Which type of fireplace is the most efficient – a floor bio-fireplace.
Additional information about biofireplaces and fuel.
The biofireplace complements the main heat source. It can be used to heat a room in the cold season.
Biofireplaces heat the room where it is installed. Even small models become an additional source of heat.
The fire is a light source, and the flame size in the bio fireplace reaches a serious size and can be adjusted by the user himself. In the event of a power cut, the biofireplace can illuminate large rooms.
Watching the flame has a positive effect on a person. It calms, gives energy, allows relaxation and recovery from stress, has a positive effect on the ability to concentrate.
When burning biofuels, only water vapor and carbon dioxide are released, which is equivalent in volume to human breathing. Dry air is the cause of respiratory, skin and eye diseases. Humidified air has a positive effect on health and immunity.
You can order bioethanol from our online store
What to keep in mind when turning off geothermal heating
- 27 Feb, 2024
- Posted by admin
- 0 Comment(s)
As the price of electricity has risen significantly, many owners of country houses and cottages are thinking about saving. One possibility is, for example, switching off geothermal heating. However, many are unaware that the system needs to be prepared for this. If the system is filled with heat transfer fluid with a freezing point below -10 ° C, it may freeze during shutdown and cause serious damage.
To avoid this problem, ethylene glycol or propylene glycol concentrate must be added to the system. In this way, it is possible to lower the freezing temperature to -36 ° C, for example, which eliminates freezing and allows the heating system to be restarted without any problems when electricity prices return to normal.
Ethylene glycol: how to dilute
- 26 Feb, 2024
- Posted by admin
- 0 Comment(s)
MasterChem Ethylene glycol is a popular heating medium with low freezing points. It is available in the form of water-glycol solutions of various concentrations, ready-to-use, or in the form of a concentrate requiring preliminary dilution.
Depending on the expected operating conditions, a reagent with a certain freezing point is selected. If you bought a ready-made water-glycol solution with a low concentration (20-35%) and its frost resistance meets the requirements of the system, then you do not need to dilute it.
If a concentrate or solution with a basic substance content of more than 40% is selected, it is recommended to dilute it before use. High concentrations of antifreeze are suitable only for the conditions of the Far North; in milder climates, dilution of the composition will be required.
Ethylene glycol: how to dilute?
The reagent should be diluted with distilled or soft water with a minimum amount of impurities (especially calcium and magnesium salts). Otherwise, hardness salts will lead to the formation of sludge, which negatively affects the cooling or heating system.
If the coolant contains anti-corrosion additives, then it is allowed to be diluted with water with a hardness not exceeding 5 mg / eq. In this case, the liquid must settle before being added to the system.
Manufacturers do not recommend pouring undiluted ethylene glycol-based antifreeze into heating or cooling systems: it has a high viscosity, which deteriorates the circulation of liquid in the pipes. In addition, it has a low heat capacity, and this drawback must be corrected by adding water.
The optimum temperatures at which the diluted reagent begins to freeze are considered to be -25 ° C or -30 ° C, for double-circuit boilers -20 ° C. For greater convenience, the table below will help determine how to dilute ethylene glycol:
| Temperatore Crystallization | Etylenglycole (l) | Water (l) |
| -20 ° C | 54 | 60 |
| -25 ° C | 60 | 40 |
| -30 ° C | 65 | 35 |
| -40 ° C | 77 | 23 |
The proportions should be determined in advance and filled with water and antifreeze separately. The filling of these fluids must be carried out in small portions one by one until the required pressure level in the system is obtained.
There is another way – filling the pipes with an already diluted coolant through the drain valve using a vibration pump. In this case, the reagent is mixed with water in the required proportions in a special container.
We will always help you calculate the volume of the required solution based on the pipe diameter and length.
Description:
Application and use:
Physical properties:
Precautionary measures:
Antifreeze is very toxic, so its use is recommended only for closed circuit designs. Application in double-circuit boilers can lead to the penetration of the coolant into the hot water supply system.
When pouring the material, it is necessary to use personal protective equipment: a mask, rubber gloves and goggles. During operation, it is necessary to completely exclude human interaction with the coolant.
Do not use the reagent as a heat carrier in places accessible to small children. Due to its sweet taste and lack of unpleasant odor, it can attract their attention, and the consequences can be dire.
If antifreeze leaks, replace all elements that have gotten into ethylene glycol, as they will subsequently become sources of toxic fumes.
Secrets and truth about washing from the manufacturer.
- 26 Feb, 2024
- Posted by admin
- 0 Comment(s)
| Ethanol-based | Methanol-based | |
| Smell | 1 (ethanol smell) | 4 (odorless) |
| Poisonous / harmful | 3 (harmful if swallowed, keep out of reach of children) | 1 (poison when ingested, protect eyes, keep out of reach of children) |
| Cost | 1 (2 times more expensive than methanol) | 5 (2 times cheaper than ethanol) |
| Detergent properties | 4 | 5 |
| Alcohol Concentration and Savings on Wiper Dilution | 4 | 5 |
Many are probably at a loss – why did the glass cleaner and its strong odor rise in price in stores! Because on May 9 a directive was adopted, according to which the sale of methanol-based glass cleaning liquid to consumers is prohibited in the EU!
The initiative is Poland, so that people do not get poisoned with methanol liquid. Again they “worried” about the people, now they will buy an expensive, almost harmless product. It turned out to be forbidden – easier than raising the level of awareness and well-being in society.
And what can I say, it is politically more profitable to use Brazilian bioethanol than cheap methanol from its eastern neighbor.
And now how to pay the average consumer, let’s understand. Immediately make a reservation – this is not an advertising article, but an informative one. Not advertising because the author of the article produces all types of glass cleaning liquid and which one the client chooses can be sold as well. We have been manufacturing glass cleaning fluid for 25 years and have good knowledge. I have identified the main points by which we consider all the advantages and disadvantages of a liquid, depending on the type of alcohol.
The main alcohols are ethanol, methanol (wood alcohol) and isopropyl alcohol. We do not consider the latter, since in the European market it occupies about 1% of the total liquid. Compare non-freezing based on methanol and ethanol.
1. Smell
Deliberately put this item in first place, based on what is important to the client.
The concept of smell is also considered by the layman, because even methanol has a “natural” smell of methanol. So, the odorless glass cleaner is based on methanol. With a smell – based on ethanol. A liquid with a lower freezing point will smell stronger (-40 smell is stronger than -15 because -40 has a higher alcohol content).
Many manufacturers add perfumes – organic compounds that give the smell of apple, lemon, frosty freshness. What they do is clog or smell stronger than alcohol to clog the smell of alcohol. That is, the smell of alcohol does not disappear, and we breathe in additional chemistry. Everyone decides whether to inhale it (in our allergic age).
To summarize – the smell leader – methanol liquid.
2. Toxic / harmful.
Methanol is a poison. Even a small amount if swallowed can cause death. Contact with eyes is also harmful. Most importantly, keep out of the reach of children. And best of all, enter a habit – open the canister and completely pour it into the tank.
Ethanol-based windshield wipers are also prohibited. The ethanol used is all denatured – with ketones, isopropyl and bitrex, which gives bitterness.
3. Cost.
Methanol is about 2 times cheaper than ethanol. As a result, ethanol-based fluid is more expensive.
4. Detergent properties.
In addition to surfactants (surfactants), the non-freezing properties of detergents depend on alcohol. And here methanol liquid wins again – menalol is better at washing glass and treats rubber wipers more carefully.
5. Alcohol concentration and economy when diluting the wiper.
I will scatter another myth. Suppose you bought a wiper with a freezing point of -40C. Outside the window there is no such frost and you dilute the -40 wiper with water in a proportion of 50/50. Now the freezing temperature is -20? – Not! – if on the basis of ethanol, then -15, if on the basis of methanol -18! The dependence of the freezing temperature / content of the sprint is not direct. And in order to produce -21 freezing point, it is necessary to use ethanol more than methanol – with the same -21 degrees. At this point, methanol liquid wins.