Water, a fundamental element in various industrial processes, serves as the primary means for heat transfer due to its unique thermophysical properties. However, the limitation associated with the freezing point of water requires the use of alternative fluids, such as antifreeze solutions, in specific scenarios.
The superiority of water as a means of heat transfer:
Water is an outstanding heat transfer fluid in industrial systems due to its high thermal conductivity and heat capacity. These properties allow for efficient heat absorption and transfer, making water an ideal medium in systems that require rapid temperature control. In addition, the low viscosity of water ensures smooth flow through pipes and channels, increasing system efficiency. Low thermal expansion reduces the risk of damage due to temperature changes. Moreover, water is readily available, inexpensive, and environmentally friendly, making it the preferred choice in many industrial applications.
Water limitation – freezing point:
Despite these benefits, water has a critical disadvantage – its freezing point at 0°C. This limitation poses a significant problem in environments exposed to sub-zero temperatures, as frozen water can cause system failure or damage.
The need for antifreeze solutions in industrial processes:
To overcome the limitation of water’s freezing point, the industry is turning to antifreeze solutions. They are vital in the food processing sector, where processes often require refrigeration. Likewise, in pharmaceutical manufacturing, maintaining specific temperatures is critical to product stability and effectiveness.
In the food and pharmaceutical industries, as well as in fire extinguishing systems, harmless aqueous solutions based on propylene glycol or glycerin are used. Solar collectors use propylene glycol-based fluids.
Natural solutions are also used in heating systems of private homes, children’s institutions, and industrial enterprises, where it is necessary to minimize the risk of harm if solutions enter the drinking system. Solutions based on propylene glycols and glycerin are safe compared to ethylene glycol solutions.
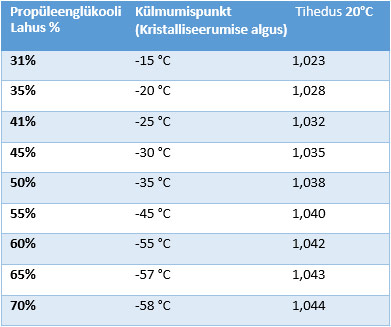
Aqueous solutions of propylene glycol developed by the MasterChem laboratory are divided into groups according to freezing point and rust protection.
Master ECO-20PRO™
Master is a patented product of MasterChem
ECO – means an aqueous solution based on propylene glycol. (Other MasterChem coolant ranges have meanings
BIO – solution of glycerin and water
EWS – ethylene glycol and water solution
-10; -20; -30; -40; -50 freezing point of aqueous solutions Celsius
NOR – aqueous solution without rust additives
PRO – aqueous solution with anti-rust additive – contains an inhibitor
for example, Master ECO-20PRO™ – an aqueous solution based on propylene glycol, does not freeze at temperatures down to -20C and contains inhibitor additives
The properties of aqueous propylene glycol solutions are indeed critical to their use as a heat transfer fluid in various applications. Let’s discuss these properties in more detail:
– Density: Aqueous solutions of propylene glycol have a higher density than water, usually 6-8% higher. This density increases with increasing propylene glycol concentration. The higher density of these solutions can affect the flow and heat transfer characteristics of systems where they are used.
– Specific Heat and Thermal Conductivity: Both the specific heat and thermal conductivity of propylene glycol solutions are lower than that of water and decrease by up to 20% with increasing propylene glycol concentration. This reduction becomes more significant at lower temperatures, especially in sub-zero conditions. This means that the solution’s ability to retain and transfer heat is reduced compared to water.
– Viscosity: Both kinematic and dynamic viscosity of aqueous solutions of propylene glycol are higher than that of water, approximately 4-5 times higher at positive temperatures. When the propylene glycol concentration is increased to about 55% (which is usually the practical limit), the viscosity can increase by a factor of 10-15, especially when the crystallization temperature drops to about -40 °C. High viscosity at lower temperatures can affect the pumpability and fluidity of the solution, which is a critical factor in system design and operation.
– These properties make aqueous solutions of propylene glycol especially useful in applications where freezing temperatures may occur, such as in HVAC systems, refrigeration, and various industrial processes. The formulation of these solutions is a balance between the required antifreeze properties and the desired heat transfer characteristics.
– It is important for engineers and technicians to consider these properties when designing and operating systems that use aqueous propylene glycol solutions to ensure optimal performance and efficiency.
The increased viscosity of aqueous solutions of propylene glycol at negative operating temperatures actually leads to significant hydraulic losses due to friction in pipelines and overcoming hydraulic resistance in all components of cooling and industrial air conditioning systems. This factor is critical in system design and operation because higher-viscosity fluids require more energy to pump, resulting in increased operating costs and potential system stress.
In addition, a decrease in the specific heat capacity and thermal conductivity of propylene glycol solutions by up to 20% compared to water requires adjustments to the operation of the system. To ensure the transfer of the required thermal power (energy), it may be necessary to increase the coolant circulation rate or other technical solutions. This can affect the efficiency of heat transfer processes and the overall performance of heating and cooling systems.
These factors become especially important in various climates where extreme temperatures can significantly affect fluid properties. When designing and operating heating and industrial air conditioning systems, engineers must consider these characteristics of propylene glycol solutions. This includes consideration of alternative system designs, such as larger pumps or pipes, to account for increased fluid viscosity and reduced heat transfer efficiency.
Thus, the use of aqueous propylene glycol solutions at extreme temperatures requires careful consideration of their thermophysical properties to ensure efficient and reliable operation of the system. This may involve a detailed analysis of system parameters and potentially more complex or expensive system designs to effectively address these issues.